PERIODIC ACID-SCHIFF, WITH OR WITHOUT DIASTASE (PAS, PASD)
Components of interest:
- Neutral polysaccharides: Glycogen (liver, skeletal muscle), starch, cellulose, chitin (fungal cell walls).
- Neutral mucosubstances (glycoproteins, known as epithelial mucins): stomach mucin, Paneth cell granules (small intestine), sialomucins from submaxillary gland mucin, small intestine mucins, fetal mucins, upper part of colonic crypts, sublingual gland.
- Basement membranes.
- Other components such as: alpha-antitrypsin, reticulin, pancreatic zymogen granules, thyroid colloid, corpora amylacea, Russell bodies.
Clinical significance:
- Differential diagnosis of tumours (e.g. adenocarcinomas will often secrete neutral mucins, glycogen granules might be present in some tumours).
- Assessment of basement membrane thickness, which can be increased pathologically (particularly in glomerular capillaries of the kidney)
- Demonstration of some species of fungi (commonly Candida albicans, Histoplasma capsulatum, Cryptococcus, Blastomyces).
- Diagnosis of other conditions such as glycogen storage diseases and alpha-antitrypsin deficiency.
Principle:
- Hydroxyl groups on adjacent carbon atoms of the carbohydrate (1,2-glycols) are oxidised by the periodic acid, creating free aldehyde groups.
- Aldehyde groups react with Schiff reagent (colourless), which results in a coloured product.
- Schiff reagent is prepared from basic fuchsin treated with sulfurous acid, which reduces the quinoid configuration that confers colour to the molecule, turning it colourless.
- Schiff reagent reacts with aldehydes in an initial reaction, followed by an aqueous rinse that removes the remaining loosely bound sulfonate groups, re-establishing the quinoid structure and therefore the colour.
- Diastase or amylase can be used to digest glycogen, breaking it down into mono- and disaccharides which are then washed out. Therefore, the PASD method demonstrates all PAS positive components except glycogen.
- PAS positive components: magenta.
- Glycogen not stained in PASD.
- Nuclei: Blue (with hematoxylin counterstain)
Controls:
- Cervix. The epithelium of the ectocervix will be PAS positive and PASD negative due to the presence of glycogen, while the endocervical glands will be positive for both due to the presence of mucins.
- Other possible controls are liver for demonstration of glycogen, and kidney for demonstration of basement membranes.
Technical notes:
- Use of different oxidants such as chromic acid or potassium permanganate is possible, but will eventually further oxidise aldehyde groups to carboxylic groups, which will not react with Schiff reagent.
- Washing in tap water after Schiff reaction is important for colour development.
- A control run omitting the periodic acid step will reveal the presence of reactive aldehyde groups already present in the tissue.
- The quality of Schiff reagent can be tested by adding a few drops of the reagent to 10 mL of formaldehyde, which should rapidly turn reddish purple.
- Variations of the technique include the use of a fast green counterstain and no hematoxylin in the demonstration of fungi.
- Historically, a sodium metabisulphite rinse was used after Schiff reagent to remove any excess background staining, however, this is unnecessary provided the rinse in tap water is adequate.
PAS AND PASD RESULTS
 |
| Liver. Pictures A and C show PASD stain with glycogen digested, while B and D show PAS stain with glycogen stained. Source: https://www.semanticscholar.org/paper/Periodic-Acid-Schiff-Staining-with-Diastase.-Fu-Campbell-Thompson/40fcdca6ab52acbbaf8bee8eb11d42b7de1e5dc3 |
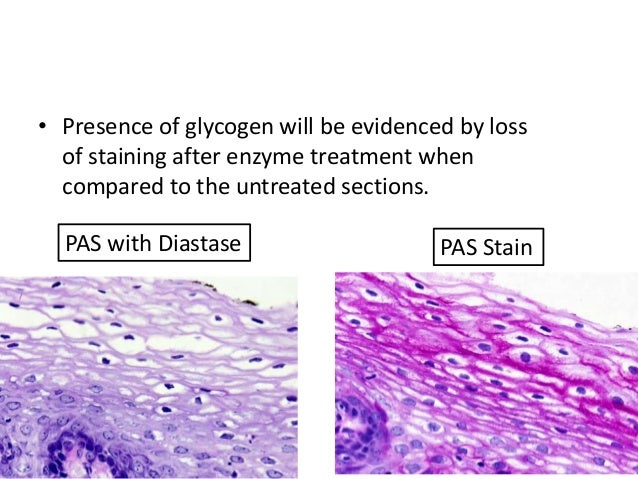 |
| Source: https://www.slideshare.net/aghara33/special-stain-in-histopathology |
 |
| Oesophageal candidiasis. Source: https://commons.wikimedia.org/wiki/File:Esophageal_candidiasis_(1)_PAS_stain.jpg |
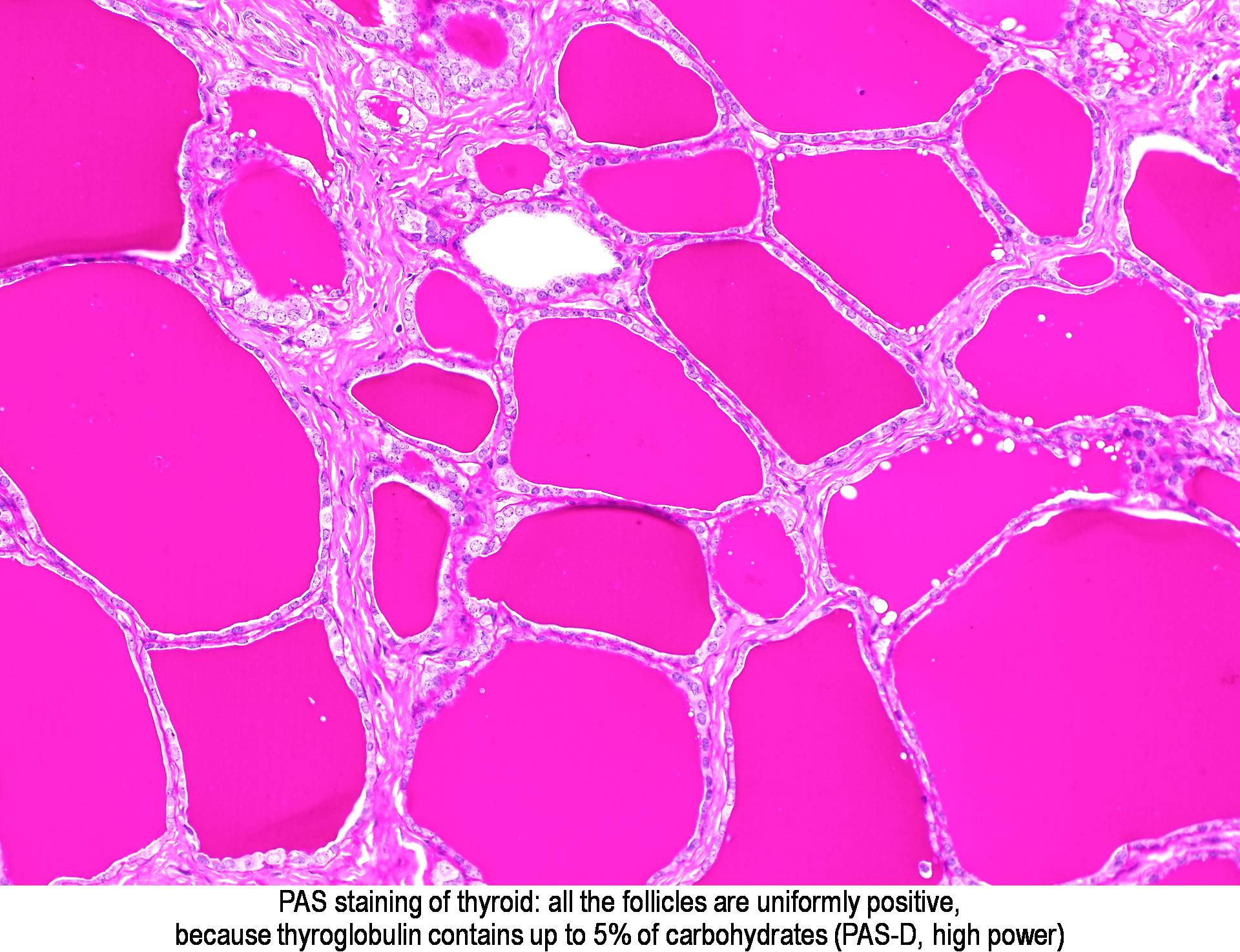 |
| Source: http://www.pathologyoutlines.com/topic/stainspas.html |
| Source: http://www.elearning.virtualpathology.leeds.ac.uk/picture.php?number=2&topic=Special%20stains&id=18&subject=Renal%20Pathology&caseid=1 |
| Source: http://www.easloffice.eu/jhep/contest/website/cat_scientific_1.html |
References:
- Suvarna, S. K., Layton, C., & Bancroft, J. D. (2018). Bancroft's Theory and Practice of Histological Techniques. Elsevier.- Kiernan, J. A. (2015). Histological and Histochemical Methods: Theory and Practice. Scion Publishing.
- Carson, F. L., & Cappellano, C. H. (2015). Histotechnology: A Self Instructional Text. ASCP.